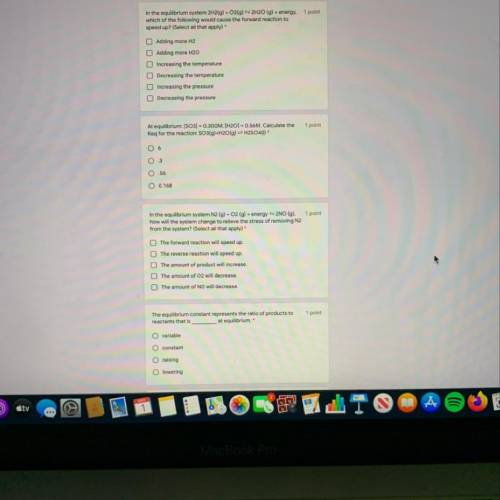
***NEED HELP***
Plus one more
5. Le Chateliers principle states that when an...

Chemistry, 05.05.2020 00:10, samyajones68
***NEED HELP***
Plus one more
5. Le Chateliers principle states that when an equilibrium system is stressed,
A. The amount of product and reactant will decrease.
B. The amount of product and reactant will increase.
C. The amount of reactants and products will change in such a way so that the stress will be relieved.
D. The forward reaction will speed up.
E. The reverse reaction will increase.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, applereams
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2

Chemistry, 21.06.2019 23:20, anggar20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Do you know the correct answer?
Questions in other subjects:







Biology, 27.06.2019 21:40

Chemistry, 27.06.2019 21:40

History, 27.06.2019 21:40

Biology, 27.06.2019 21:40






