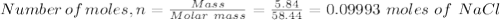A student has a mixture of salt (NaCl) and sugar (C12H22O11). To determine the percentage, the student measures out 5.84 grams of the mixture and dissolves this in 100.0 mL of water. The student knows that aqueous salt with aqueous silver nitrate (AgNO3) to form solid silver chloride (AgCl). The student determines that sugar will not react with silver nitrate. How many mL of 1.0 M AgNO3 would be required to precipitate 5.84 grams of NaCl?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:10, GreatBaconGamer
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 17:00, davisnaziyahovz5sk
The arrangement of particles is most ordered in a sample of
Answers: 1

Chemistry, 22.06.2019 17:30, llamasking
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2
Do you know the correct answer?
A student has a mixture of salt (NaCl) and sugar (C12H22O11). To determine the percentage, the stude...
Questions in other subjects:


Chemistry, 18.11.2020 21:10




Mathematics, 18.11.2020 21:10

Mathematics, 18.11.2020 21:10



English, 18.11.2020 21:10