
Chemistry, 05.05.2020 03:03, colexie7410
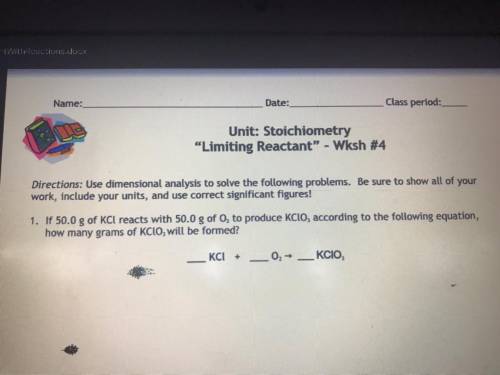
If 50.0g of KCl reacts with 50.0g of O2 to produce KClO3 according to the following equation, how many grams of KClO3 will be formed?
_KCl+_O2—>_KCIO3

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, annan65
Jewelweed, a flowering plant, has seed pods that burst open when touched and forcefully eject their seeds. these structures are favorable because they a. can cause genetic changes to occur. b. prevent germination within the seed pod. c. aid in the dispersal of the species. d. attract insects that aid in pollination.
Answers: 3


Do you know the correct answer?
If 50.0g of KCl reacts with 50.0g of O2 to produce KClO3 according to the following equation, how ma...
Questions in other subjects:


Mathematics, 29.06.2019 00:30

Mathematics, 29.06.2019 00:30





Mathematics, 29.06.2019 00:30

English, 29.06.2019 00:30






