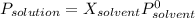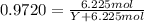Chemistry, 05.05.2020 04:23, NylaJohn29
The common laboratory solvent ethanol is often used to purify substances dissolved in it. The vapor pressure of ethanol , CH3CH2OH, is 54.68 mm Hg at 25 °C. In a laboratory experiment, students synthesized a new compound and found that when 32.83 grams of the compound were dissolved in 286.8 grams of ethanol, the vapor pressure of the solution was 53.15 mm Hg. The compound was also found to be nonvolatile and a non-electrolyte. What is the molecular weight of this compound? ethanol = CH3CH2OH = 46.07 g/mol.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:20, sarinaneedshelp01
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1


Chemistry, 22.06.2019 19:00, HaydenSturgis1
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 22.06.2019 23:30, jade468
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1
Do you know the correct answer?
The common laboratory solvent ethanol is often used to purify substances dissolved in it. The vapor...
Questions in other subjects:

Mathematics, 26.05.2021 08:30

History, 26.05.2021 08:30


History, 26.05.2021 08:30

Chemistry, 26.05.2021 08:30


Mathematics, 26.05.2021 08:30

Mathematics, 26.05.2021 08:30

Computers and Technology, 26.05.2021 08:30