
Consider the two reduction half-reactions: Na+(aq) + e− LaTeX: \longrightarrow⟶ Na(s) Eo = −2.71 V Cl2(g) + 2 e− LaTeX: \longrightarrow⟶ 2 Cl−(aq) Eo = 1.36 VUse the electrode potentials above to calculate Eocell and ∆Gorxn for the reaction below, and determine if it is the reaction for a voltaic cell or an electrolytic cell. 2 Na+(aq) + 2 Cl−(aq) LaTeX: \longrightarrow⟶2 Na(s) + Cl2(g)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, kaliyab191
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2


Chemistry, 23.06.2019 05:00, skylarjohnson2683
Match the term to its description match term definition chemical energy a) internal energy caused by vibrations of atoms and molecules electrical energy b) electromagnetic energy that travels in waves radiant energy c) the movement of an electrical charge thermal energy d) potential energy stored in the bonds between atoms
Answers: 1

Chemistry, 23.06.2019 10:00, anonymous176
An uncovered pot of water lies out in the sun. which statements correctly describe what happens at the surface of the liquid water? 1. the vapor pressure remains constant regardless of the water temperature. 2. the vapor pressure is produced by water molecules that have evaporated. 3. the vapor pressure increases as the sun heats the water in the pot. 4. evaporation stops once the vapor pressure reaches a certain point. 5. evaporation and condensation both occur on the liquid’s surface.
Answers: 3
Do you know the correct answer?
Consider the two reduction half-reactions: Na+(aq) + e− LaTeX: \longrightarrow⟶ Na(s) Eo = −2.71 V C...
Questions in other subjects:

Biology, 04.07.2019 10:30




English, 04.07.2019 10:30

Social Studies, 04.07.2019 10:30

Social Studies, 04.07.2019 10:30

History, 04.07.2019 10:30


Mathematics, 04.07.2019 10:30


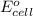 = -4.07v
= -4.07v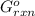 = +785.510 KJ
= +785.510 KJ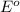 and Δ
and Δ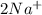 +
+ 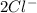 →
→ 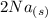 +
+ 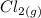
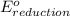 - Δ
- Δ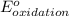 = -2.71v - 1.36v = -4.07V
= -2.71v - 1.36v = -4.07V



