Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:00, montimcdaniel
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1

Chemistry, 22.06.2019 23:00, hailey5campbelp7d1c0
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2

Chemistry, 22.06.2019 23:00, poolwaterisgross
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1
Do you know the correct answer?
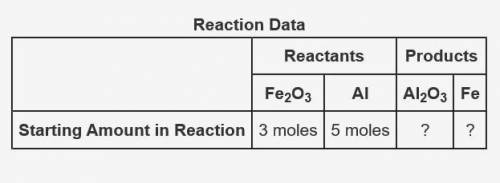
The following data was collected when a reaction was performed experimentally in the laboratory.
Questions in other subjects:



Computers and Technology, 16.08.2021 20:10

Mathematics, 16.08.2021 20:10


English, 16.08.2021 20:10

Mathematics, 16.08.2021 20:10

Mathematics, 16.08.2021 20:10

Chemistry, 16.08.2021 20:10