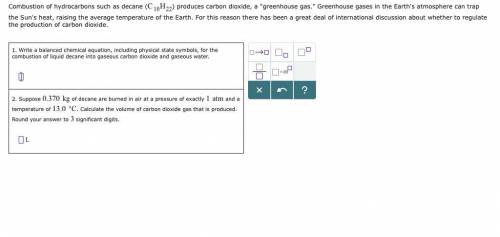
Combustion of hydrocarbons such as decane () produces carbon dioxide, a "greenhouse gas." Greenhouse gases in the Earth's atmosphere can trap the Sun's heat, raising the average temperature of the Earth. For this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide. 1. Write a balanced chemical equation, including physical state symbols, for the combustion of liquid decane into gaseous carbon dioxide and gaseous water. 2. Suppose of decane are burned in air at a pressure of exactly and a temperature of . Calculate the volume of carbon dioxide gas that is produced. Round your answer to significant digits.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:40, georgehall3027
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 10:30, shaylawaldo11
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3

Do you know the correct answer?
Combustion of hydrocarbons such as decane () produces carbon dioxide, a "greenhouse gas." Greenhouse...
Questions in other subjects:

Biology, 10.09.2020 01:01

Physics, 10.09.2020 01:01

Mathematics, 10.09.2020 01:01

Mathematics, 10.09.2020 01:01

Mathematics, 10.09.2020 01:01

Geography, 10.09.2020 01:01

Biology, 10.09.2020 01:01

Mathematics, 10.09.2020 01:01

Social Studies, 10.09.2020 01:01

English, 10.09.2020 01:01