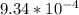Chemistry, 05.05.2020 07:20, stphdrn4347
Onsider the reversible dissolution of lead(II) chloride. P b C l 2 ( s ) − ⇀ ↽ − P b 2 + ( a q ) + 2 C l − ( a q ) PbClX2(s)↽−−⇀PbX2+(aq)+2ClX−(aq) Suppose you add 0.2393 g of P b C l 2 ( s ) PbClX2(s) to 50.0 mL of water. When the solution reaches equilibrium, you find that the concentration of P b 2 + ( a q ) PbX2+(aq) is 0.0159 M and the concentration of C l − ( a q ) ClX−(aq) is 0.0318 M. What is the value of the equilibrium constant, Kc, for the dissolution of P b C l 2 PbClX2?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, youngchapo813p8d9u1
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 14:30, srutkowske1489
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1

Chemistry, 22.06.2019 16:00, winnie45
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 22.06.2019 18:00, darrell1168
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3
Do you know the correct answer?
Onsider the reversible dissolution of lead(II) chloride. P b C l 2 ( s ) − ⇀ ↽ − P b 2 + ( a q ) + 2...
Questions in other subjects:




Mathematics, 10.10.2020 23:01



Advanced Placement (AP), 10.10.2020 23:01





 ⇌
⇌ 
![[Pb^{2+}]](/tpl/images/0634/7278/0acfd.png) = 0.0159 M
= 0.0159 M
![[Cl^-]](/tpl/images/0634/7278/0726e.png) = 0.0318 M
= 0.0318 M

 Number of moles of PbCl₂
Number of moles of PbCl₂ 


![K_c=\frac{[Pb^{2+}][Cl^-]}{[PbCl_2]} \\\\](/tpl/images/0634/7278/c4946.png)