
Chemistry, 05.05.2020 08:19, iwannabewinston
Part A - Which numbers are used? Consider the balanced chemical equation that follows. You are asked to determine how many moles of water you can produce from 4.0 molmol of hydrogen and excess oxygen. (Excess oxygen means that so much oxygen is available it will not run out.) Which of the numbers that appear in the balanced chemical equation below are used to perform this calculation? 2H2(g)+O2(g)→2H2O(l)2H2(g)+O2(g)→2H 2O(l) View Available Hint(s)

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:00, issachickadi
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 14:30, srutkowske1489
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1

Do you know the correct answer?
Part A - Which numbers are used? Consider the balanced chemical equation that follows. You are asked...
Questions in other subjects:

Mathematics, 18.04.2021 21:30

History, 18.04.2021 21:30

Mathematics, 18.04.2021 21:30

Mathematics, 18.04.2021 21:30


English, 18.04.2021 21:30


Mathematics, 18.04.2021 21:30



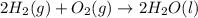
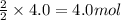 of water.
of water.



