
Chemistry, 05.05.2020 13:27, Gghbhgy8716
You burn a 15g jellybean to warm 50 mL of water, which increases the temperature of the water 25 °C. How many calories of heat are transferred from the jellybean to the water? Assume there is no heat loss.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, willcohen42
150.0 grams of asf3 were reacted with 180.0 g of ccl4 to produce ascl3 and ccl2f2. if the actual yield of ccl2f2 was 127 g, what is the percent yield?
Answers: 2


Chemistry, 22.06.2019 17:30, sheazy3709
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

Chemistry, 22.06.2019 21:30, sullivanjakob
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1
Do you know the correct answer?
You burn a 15g jellybean to warm 50 mL of water, which increases the temperature of the water 25 °C....
Questions in other subjects:







Mathematics, 25.09.2019 01:00


English, 25.09.2019 01:00


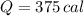


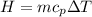
 of water= 4.18J/g/°C
of water= 4.18J/g/°C



