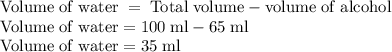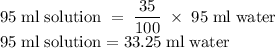Answers: 2
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 06:00, bvbbridesmaid5519
Is the flow of energy during vaporizing more like the flow during melting or during freezing
Answers: 1

Chemistry, 23.06.2019 06:00, wirchakethan23
When hydrogen peroxide (h2o2) is added to potassium iodide (ki) solution, the hydrogen peroxide decomposes into water (h2o) and oxygen (o2). the chemical equation for the decomposition reaction is: 2h2o2—> 2h2o + o2. what is the role of the potassium iodide in this reaction? a. reactant. b. product. c. precipitate. d. catalyst.
Answers: 1

Chemistry, 23.06.2019 07:00, asims13
The following transition occurs at a molecular level for a substance. what transition corresponds to this change in microscopic structure? the carbon dioxide molecules on the left are in a regular, tightly packed pattern. after heating, it becomes much lower density. a. melting b. boiling c. sublimation d. freezing
Answers: 1

Chemistry, 23.06.2019 07:00, kotetravels10
0.88 moles of n2o5 (g) was placed in a sealed 1.00 l vessel. calculate the equilibrium concentration of n2o5. no2, and o2 and the equilibrium constant after equilibrium has been reached by 65.0% of the n2o5 decomposing.
Answers: 1
Do you know the correct answer?
An aqueous solution is 65% (v/v) rubbing alcohol. How many millilitres of water are in a 95-ml sampl...
Questions in other subjects:

Biology, 04.08.2019 01:00






Social Studies, 04.08.2019 01:00


History, 04.08.2019 01:00