
Chemistry, 05.05.2020 15:17, klivingston1012
How many moles of sulfuric acid are required to make (4.0x10^2) mL of (1.140x10^-1) mol/L solution?
* Express your answer in scientific notation to the correct number of significant digits. Remember th:
scientific notation has one non-zero number to the left of the decimal point (i. e.: 1.23 x 104).
Note: Your answer is assumed to be reduced to the highest power possible.
Your
X10
Answer
units

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, XxrazorxX11
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 09:00, heids17043
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 15:40, alleshia2007
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 16:10, sierram298
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1
Do you know the correct answer?
How many moles of sulfuric acid are required to make (4.0x10^2) mL of (1.140x10^-1) mol/L solution?<...
Questions in other subjects:


Mathematics, 03.12.2020 19:30

German, 03.12.2020 19:30


Social Studies, 03.12.2020 19:30

History, 03.12.2020 19:30


English, 03.12.2020 19:30



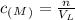 where n is the moles of the solute, and V is the volume of the solution in liters (L).
where n is the moles of the solute, and V is the volume of the solution in liters (L).

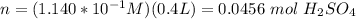
 (rounded)
(rounded)



