Chemistry, 05.05.2020 14:58, mccay5016987
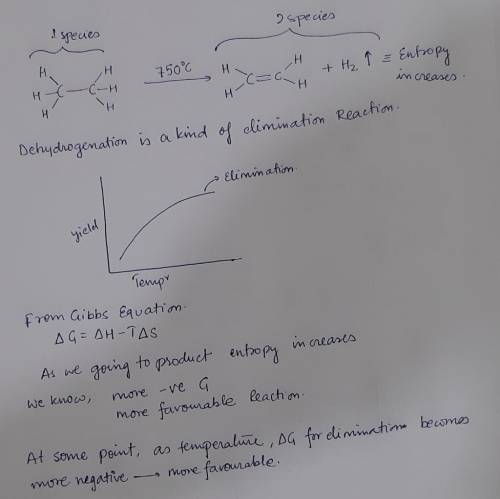
6. At high temperatures, alkanes can undergo dehydrogentation to produce alkenes. This reaction is used industrially to prepare ethylene while simultaneously serving as a source of hydrogen gas. Explain why dehydrogenation only works at high temperatures using approximately 20 words or less. (5 poin

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 15:10, strodersage
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 23.06.2019 00:30, joshsmith2022
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1
Do you know the correct answer?
6. At high temperatures, alkanes can undergo dehydrogentation to produce alkenes. This reaction is u...
Questions in other subjects:

Mathematics, 07.01.2021 01:00

Computers and Technology, 07.01.2021 01:00

English, 07.01.2021 01:00

Mathematics, 07.01.2021 01:00

Chemistry, 07.01.2021 01:00


Health, 07.01.2021 01:00

Computers and Technology, 07.01.2021 01:00

Mathematics, 07.01.2021 01:00

Chemistry, 07.01.2021 01:00