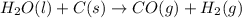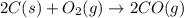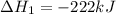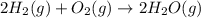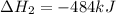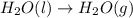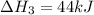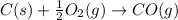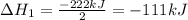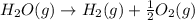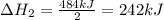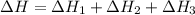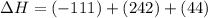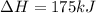Chemistry, 05.05.2020 16:29, shawn20034
2C(s)+O2(g)2H2(g)+O2(g)H2O(l)→2CO(g )→2H2O(g)→H2O(g)ΔHΔHΔH=−222kJ=−484k J=+44kJ Use the thermochemical data above to calculate the change in enthalpy for the reaction below. H2O(l)+C(s)→CO(g)+H2(g)

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:00, hjgjlgkjg
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 16:30, ddmoorehouseov75lc
Correct relationship between molecular formula and empirical formula
Answers: 1
Do you know the correct answer?
2C(s)+O2(g)2H2(g)+O2(g)H2O(l)→2CO(g )→2H2O(g)→H2O(g)ΔHΔHΔH=−222kJ=−484k J=+44kJ Use the thermochemic...
Questions in other subjects:




History, 24.06.2019 02:30

History, 24.06.2019 02:30





Biology, 24.06.2019 02:30