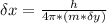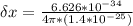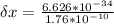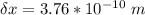A student is examining a bacterium under the microscope. The E. coli bacterial cell has a mass of m = 0.500 fg (where a femtogram, fg, is 10−15g) and is swimming at a velocity of v = 7.00 μm/s , with an uncertainty in the velocity of4.00 %.. E. coli bacterial cells are around 1 μm ( 10−6 m) in length. The student is supposed to observe the bacterium and make a drawing. However, the student, having just learned about the Heisenberg uncertainty principle in physics class, complains that she cannot make the drawing. She claims that the uncertainty of the bacterium's position is greater than the microscope's viewing field, and the bacterium is thus impossible to locate.
What is the uncertainty of the position of the bacterium?
Express your answer with the appropriate units.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:40, allyyzz
Astudent is given a sample of a blue copper sulfate hydrate. he weighs the sample in a dry covered porcelain crucible and got a mass of 23.875 g for the crucible, lid, and sample. the mass of the empty crucible and lid was found earlier to be 22.652 g. he then heats the crucible to expel the water of hydration, keeping the crucible at red heat for 10 minutes with the lid slightly ajar. on colling, he finds the mass of crucible, lid, and contents to be 23.403 g. the sample was changed in the process to very light clue anhydrous cuso4. if there are again 100.0 g of hydrate, how many grams of cuso4 are in it? how many moles of cuso4? (hint: molar mass of cuso4 = 159.6 g / mole. what per cent of the hydrate is cuso4? you may convert the mass of cuso4 to moles.)
Answers: 3


Chemistry, 22.06.2019 12:20, missayers172
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 17:00, abbygailgo674
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1
Do you know the correct answer?
A student is examining a bacterium under the microscope. The E. coli bacterial cell has a mass of m...
Questions in other subjects:

Mathematics, 29.06.2019 06:40


Spanish, 29.06.2019 06:40



Biology, 29.06.2019 06:40

Mathematics, 29.06.2019 06:40


Mathematics, 29.06.2019 06:40

Mathematics, 29.06.2019 06:40


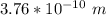
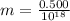
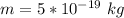
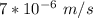
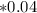
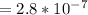
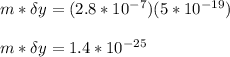
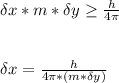
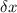 = uncertainty in the position
= uncertainty in the position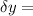 uncertainty in the velocity
uncertainty in the velocity