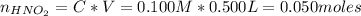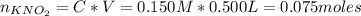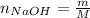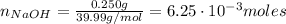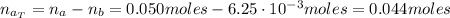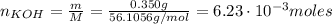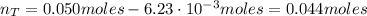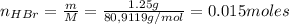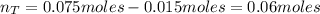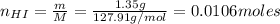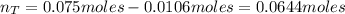A 500.0-mL buffer solution is 0.100 M in HNO2 and 0.150 M in KNO2. Part A Determine whether or not 250 mgNaOH would exceed the capacity of the buffer to neutralize it. Determine whether or not 250 would exceed the capacity of the buffer to neutralize it. yes no Request Answer Part B Determine whether or not 350 mgKOH would exceed the capacity of the buffer to neutralize it. Determine whether or not 350 would exceed the capacity of the buffer to neutralize it. yes no Request Answer Part C Determine whether or not 1.25 gHBr would exceed the capacity of the buffer to neutralize it. Determine whether or not 1.25 would exceed the capacity of the buffer to neutralize it. yes no Request Answer Part D Determine whether or not 1.35 gHI would exceed the capacity of the buffer to neutralize it. Determine whether or not 1.35 would exceed the capacity of the buffer to neutralize it. yes no

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:20, dgadam7495
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1


Chemistry, 22.06.2019 23:00, Mynameismath
Which type of intermolecular attractions holds ammonia molecules together with other ammonia molecules?
Answers: 3
Do you know the correct answer?
A 500.0-mL buffer solution is 0.100 M in HNO2 and 0.150 M in KNO2. Part A Determine whether or not 2...
Questions in other subjects:

Mathematics, 02.10.2019 04:00

Mathematics, 02.10.2019 04:00

Mathematics, 02.10.2019 04:00

Biology, 02.10.2019 04:00

English, 02.10.2019 04:00


English, 02.10.2019 04:00

English, 02.10.2019 04:00