
Chemistry, 05.05.2020 17:25, morganhines181
Be sure to answer all parts. The balanced equation for the reaction of aluminum metal and chlorine gas is 2Al(s) + 3Cl2(g) → 2AlCl3(s) Assume that 0.80 g Al is mixed with 0.23 g Cl2. (a) What is the limiting reactant? Cl2 Al (b) What is the maximum amount of AlCl3, in grams, that can be produced? g AlCl3

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:40, gonzaleze18
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2


Chemistry, 22.06.2019 20:00, montimcdaniel
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1
Do you know the correct answer?
Be sure to answer all parts. The balanced equation for the reaction of aluminum metal and chlorine g...
Questions in other subjects:


English, 01.03.2021 20:20


Social Studies, 01.03.2021 20:20



History, 01.03.2021 20:20



Social Studies, 01.03.2021 20:20


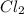 is the limiting reagent
is the limiting reagent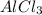 will be produced.
will be produced.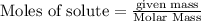

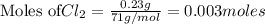
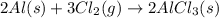
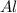
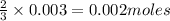 of
of 




