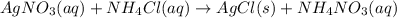Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, XxrazorxX11
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 06:00, palomaresmitchelle
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 22:30, wpatskiteh7203
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1
Do you know the correct answer?
A chemist dissolves silver nitrate (AgNO₃) in water. He also dissolves ammonium chloride (NH₄Cl) in...
Questions in other subjects:

Mathematics, 19.11.2019 23:31

Mathematics, 19.11.2019 23:31





Physics, 19.11.2019 23:31

Chemistry, 19.11.2019 23:31