
Chemistry, 05.05.2020 17:07, Natavia3402
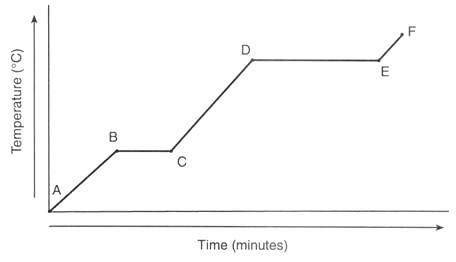
A 47.7 g chunck of ice at -58.0 °C is heated until it completely melts. Find the total amount of heat in joules for this process to occur. = 2.09J/g°C
= 2.03 J/g°C
= 4.184 J/g°C
∆ = 334 J/g
∆ = 2260 J/g
What is the total amount of energy needed to overall kilojoules (three significant figures)?
A. 21.7 kJ
B. 5.78 kJ
C. 15.9 kJ
D. 10.1

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, AIhunter2884
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 05:30, NorbxrtThaG
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 06:30, 91miketaylor
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Do you know the correct answer?
A 47.7 g chunck of ice at -58.0 °C is heated until it completely melts. Find the total amount of hea...
Questions in other subjects:


History, 09.07.2019 19:30


Mathematics, 09.07.2019 19:30



History, 09.07.2019 19:30


History, 09.07.2019 19:30







