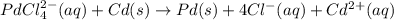Chemistry, 05.05.2020 18:31, cdradlet2001
A voltaic cell that uses the reaction PdCl42−(aq)+Cd(s) → Pd(s)+4Cl−(aq)+Cd2+(aq) has a measured standard cell potential of +1.03 V. You may want to reference (Pages 860 - 867) Section 20.4 while completing this problem. Part A Write the half-cell reaction at the cathode.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 18:00, sleimanabir
Balance this equation: n2 + h2 > nh3, write the following molar ratios: n2 / n2 / nh3 h2 /
Answers: 1

Chemistry, 22.06.2019 00:30, catdog5225
Drive down any three characteristic of modern periodic table
Answers: 1
Do you know the correct answer?
A voltaic cell that uses the reaction PdCl42−(aq)+Cd(s) → Pd(s)+4Cl−(aq)+Cd2+(aq) has a measured sta...
Questions in other subjects:


Biology, 15.07.2019 11:30

Biology, 15.07.2019 11:30

Mathematics, 15.07.2019 11:30



Social Studies, 15.07.2019 11:30


Biology, 15.07.2019 11:30

English, 15.07.2019 11:30