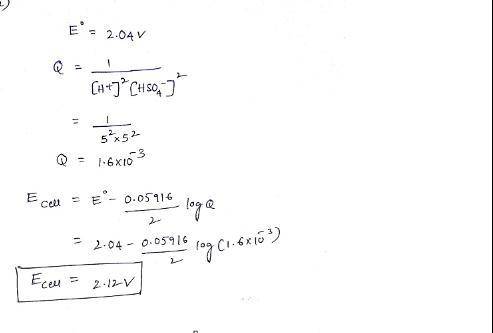Chemistry, 05.05.2020 18:14, kenleighbrooke67
The overall reaction in the lead storage battery is
Pb(s) + PbO2(s) + 2H1(aq) + 2HSO42(aq) > 2PbSO4(s) + 2H2O(l)
Calculate % at 258C for this battery when [H2SO4] 5 4.5 M; that is, [H1] 5 [HSO42] 5 4.5 M. At 258C, %8 5 2.04 V for the lead storage battery.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, DarcieMATHlin2589
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1


Chemistry, 22.06.2019 12:00, carvajalj2520
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 23:30, emmalado45
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1
Do you know the correct answer?
The overall reaction in the lead storage battery is
Pb(s) + PbO2(s) + 2H1(aq) + 2HSO42(...
Pb(s) + PbO2(s) + 2H1(aq) + 2HSO42(...
Questions in other subjects:

Mathematics, 25.02.2020 03:43


Mathematics, 25.02.2020 03:43

Health, 25.02.2020 03:43








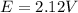
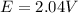
![Q = \frac{1}{[[HSO_4^-]^2 [H^+] ^2]}](/tpl/images/0641/1298/ac965.png)
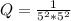

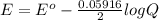
![= 2.04 - [\frac{0.05916}{2} log (1.6*10^{-3})]](/tpl/images/0641/1298/8888a.png)