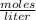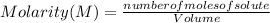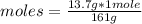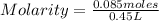Chemistry, 05.05.2020 19:24, beckyroof5158
Find the molarity of a 450 mL solution containing 13.7 g of ZnSO4.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, tifftiff22
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 05:40, yah2muchh
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 08:20, pilarmonsivais
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Do you know the correct answer?
Find the molarity of a 450 mL solution containing 13.7 g of ZnSO4....
Questions in other subjects:

Mathematics, 23.10.2020 18:20


History, 23.10.2020 18:20

Mathematics, 23.10.2020 18:20


Mathematics, 23.10.2020 18:20