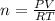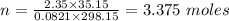Chemistry, 05.05.2020 21:21, janiyahmcgolley
Methane burns in the presence of oxygen to produce carbon dioxide and water in the following reaction:
CH4 (s) + 2 O2 (g) --> CO2 (g) + 2 H2O (l)
What mass of methane (in grams) will require 35.15 L of oxygen to fully react? The pressure is 2.35 atm and the temperature is 15 °C. (R = 0.0821 L atm/mol K)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, milesjreece3939
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1


Chemistry, 22.06.2019 17:30, rollercoasterbuddies
Why is the melting of ice a physical change ?
Answers: 1

Chemistry, 22.06.2019 21:30, sarah192002
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1
Do you know the correct answer?
Methane burns in the presence of oxygen to produce carbon dioxide and water in the following reactio...
Questions in other subjects:

Mathematics, 24.11.2020 23:00

Mathematics, 24.11.2020 23:00

Biology, 24.11.2020 23:00


Mathematics, 24.11.2020 23:00

Mathematics, 24.11.2020 23:00


Mathematics, 24.11.2020 23:00


Mathematics, 24.11.2020 23:00