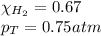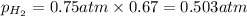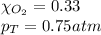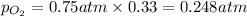Chemistry, 05.05.2020 22:21, ayoismeisjjjjuan
Solve the following, show all work and units for your calculation: Let's say we have a mixture of hydrogen gas
(H2), and oxygen gas (O2). The mixture contains 6.7 mol hydrogen gas and 3.3 mol oxygen gas. The mixture is
in a 300 L container at 273 K and the total pressure of the gas mixture is 0.75 atm. What is the partial
pressure for each gas?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, rileyeddins1010
List four observations that indicate that a chemical reaction may be taking place
Answers: 1


Chemistry, 22.06.2019 15:00, NatalieKnows
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Do you know the correct answer?
Solve the following, show all work and units for your calculation: Let's say we have a mixture of hy...
Questions in other subjects:

Social Studies, 04.07.2019 21:40

Chemistry, 04.07.2019 21:40




Mathematics, 04.07.2019 21:40

Social Studies, 04.07.2019 21:40


Social Studies, 04.07.2019 21:40


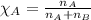
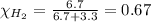
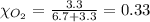
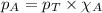
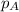 = partial pressure of substance
= partial pressure of substance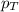 = total pressure
= total pressure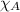 = mole fraction of substance
= mole fraction of substance