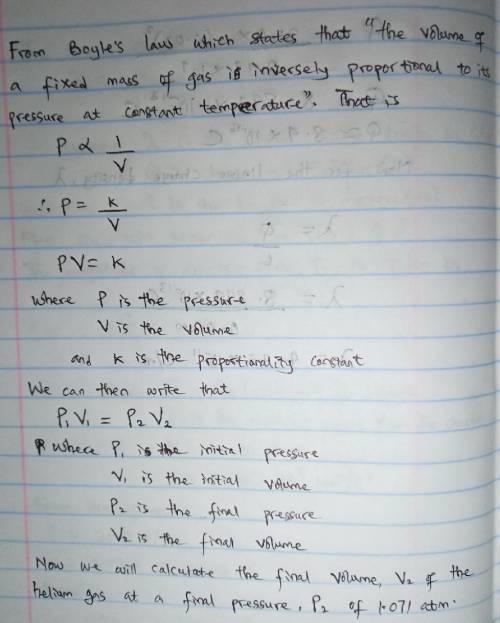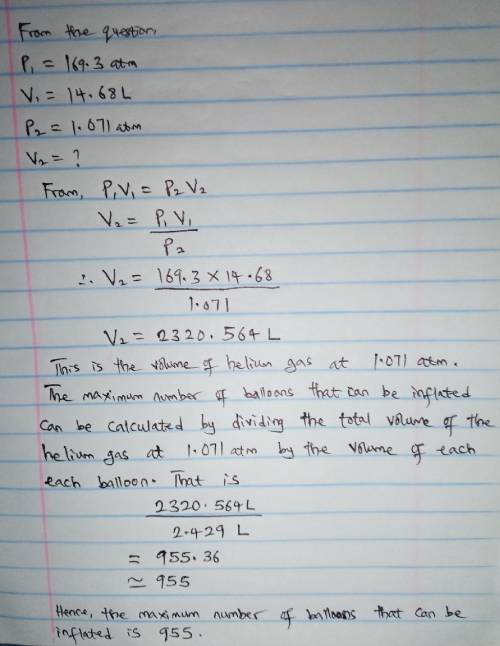Chemistry, 05.05.2020 22:21, mvazquez298
A cylinder containing 14.68 L of helium gas at a pressure of 169.3 atm is to be used to fill toy balloons to a pressure of 1.071 atm. Each inflated balloon has a volume of 2.429 L. What is the maximum number of balloons that can be inflated? Report your answer to 1 decimal place. (Remember that 14.68 L of helium at 1.071 atm will remain in the exhausted (empty) cylinder)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, mimireds5419
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3


Do you know the correct answer?
A cylinder containing 14.68 L of helium gas at a pressure of 169.3 atm is to be used to fill toy bal...
Questions in other subjects:



Mathematics, 04.02.2021 05:10


History, 04.02.2021 05:10

Biology, 04.02.2021 05:10

English, 04.02.2021 05:10

Mathematics, 04.02.2021 05:10

Biology, 04.02.2021 05:10

Mathematics, 04.02.2021 05:10