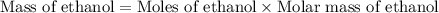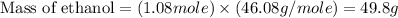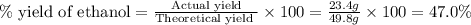Glucose (C6H12O6)(C6H12O6) can be fermented to yield ethanol (CH3CH2OH)(CH3CH2OH) and carbon dioxide (CO2).
C6H12O6⟶2CH3CH2OH+2CO2
The molar mass of glucose is 180.15 g/mol,180.15 g/mol, the molar mass of ethanol is 46.08 g/mol,46.08 g/mol, and the molar mass of carbon dioxide is 44.01 g/mol.
a) What is the theoretical yield (in grams) of ethanol from the fermentation of 97.5 g of glucose?
b) If the reaction produced 23.4 g of ethanol, what was the percent yield?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, andybiersack154
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 21.06.2019 22:30, erikloza12pdidtx
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 02:00, issachickadi
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 02:20, dgadam7495
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1
Do you know the correct answer?
Glucose (C6H12O6)(C6H12O6) can be fermented to yield ethanol (CH3CH2OH)(CH3CH2OH) and carbon dioxide...
Questions in other subjects:

Mathematics, 17.05.2020 00:57



English, 17.05.2020 00:57

Mathematics, 17.05.2020 00:57


World Languages, 17.05.2020 00:57

Mathematics, 17.05.2020 00:57


Spanish, 17.05.2020 00:57


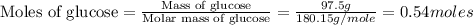
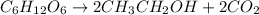
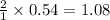 mole of ethanol
mole of ethanol