
Chemistry, 05.05.2020 22:04, williamrobinson93
Use standard entropies to calculate δs∘rxn for the balanced chemical equation: 2pcl3(g)+o2(g)→2pocl3(l) substance s∘(j/mol⋅k) pocl3(l) 222.5 pocl3(g) 325.5 pcl3(l) 217.1 pcl3(g) 311.8 o2(g) 205.2

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, vanessa051266
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 17:30, ander67061
Air can be considered a mixture. which statement does not explain why?
Answers: 1

Do you know the correct answer?
Use standard entropies to calculate δs∘rxn for the balanced chemical equation: 2pcl3(g)+o2(g)→2pocl3...
Questions in other subjects:




Biology, 24.08.2019 23:50

Geography, 24.08.2019 23:50

English, 24.08.2019 23:50

History, 24.08.2019 23:50

Social Studies, 24.08.2019 23:50

Mathematics, 24.08.2019 23:50

Mathematics, 24.08.2019 23:50


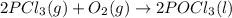
![\Delta S^o_{rxn}=[(2\times \Delta S^o_{(CO_2(g))})]-[(1\times \Delta S^o_{(O_2(g))})+(2\times \Delta S_{(PCl_3(g))})]](/tpl/images/0643/0247/f3c7b.png)
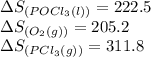
![\Delta S^o_{rxn}=[(2\times (222.5))]-[(1\times 205.2)+(2\times (311.8))]](/tpl/images/0643/0247/90f90.png)




