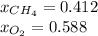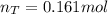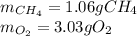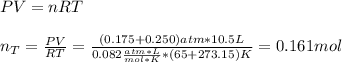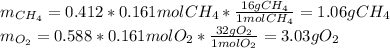Chemistry, 06.05.2020 00:28, andrwisawesome0
The partial pressure of CH4(g) is 0.175 atm, and the partial pressure of O2(g) is 0.250 atm in a mixture of the two gases. The mixture occupies a volume of 10.5 L at 65 oC. Solve all three parts of the question. 1) The mole fraction of CH4(g) is , and the mole fraction of O2(g) is . 2) The total number of moles of gas in the mixture is . 3) There are grams of CH4(g) and grams of O2(g) in the mixture.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:10, GreatBaconGamer
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 09:20, pandaman632
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 14:30, Hannahmiller3773
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 22.06.2019 22:30, darceline1574
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
Do you know the correct answer?
The partial pressure of CH4(g) is 0.175 atm, and the partial pressure of O2(g) is 0.250 atm in a mix...
Questions in other subjects:





Mathematics, 23.01.2021 01:50



Mathematics, 23.01.2021 01:50

Mathematics, 23.01.2021 01:50

Mathematics, 23.01.2021 01:50