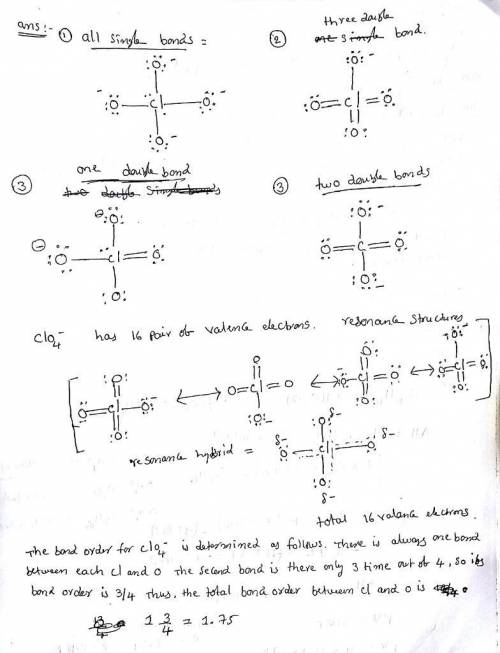
Erchlorates are powerful oxidizing agents used in fireworks, flares, and space shuttle booster rockets. Lewis structures for the perchlorate ion (ClO4−) can be drawn with all single bonds or with one, two, or three double bonds. Draw each of these possible resonance forms, including any nonbonding electrons. Include the values of any nonzero formal charges. Use formal charges to determine the most important resonance structure and calculate its average bond order.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:50, Catracho3619
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 01:00, deaishaajennings123
What is the equilibrium constant of aa+bb=cc+dd
Answers: 1

Chemistry, 22.06.2019 04:00, fantasticratz2
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2
Do you know the correct answer?
Erchlorates are powerful oxidizing agents used in fireworks, flares, and space shuttle booster rocke...
Questions in other subjects:







Physics, 17.12.2019 20:31