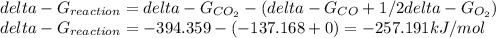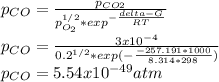Chemistry, 06.05.2020 00:21, Jenniferojeda2002
Hat is the pressure of CO(g) in equilibrium with the CO2(g) and O2(g) in the atmosphere at 25 C? The partial pressure of O2(g) is 0.2 bar and the partial pressure of CO2(g) is 3 * 10-4 bar. CO is extremely poisonous because it forms a very strong complex with hemoglobin. Should you worry?

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, ethanw8973
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3


Chemistry, 23.06.2019 14:00, RichardKing2376
Tinererining 01: 57: 44 which statement correcte describes the actual veld and the theoretical yield of a reaction? textual vec is calculated to the reactant amounts but the theoretical yeld must be measured for each instance of a the actual vec is calculated from the amount of the limiting reactant and the theoretical yield is calculated from the 发公主 the actual weld depends on the reaction centers, but the theoretical yield and only with reactant amounts the actual vele represents the maximum weld possible and the theoretical yield assumes perfect reaction conditions save and ext e அட
Answers: 2
Do you know the correct answer?
Hat is the pressure of CO(g) in equilibrium with the CO2(g) and O2(g) in the atmosphere at 25 C? The...
Questions in other subjects:

Mathematics, 07.01.2020 02:31

Business, 07.01.2020 02:31




Business, 07.01.2020 02:31