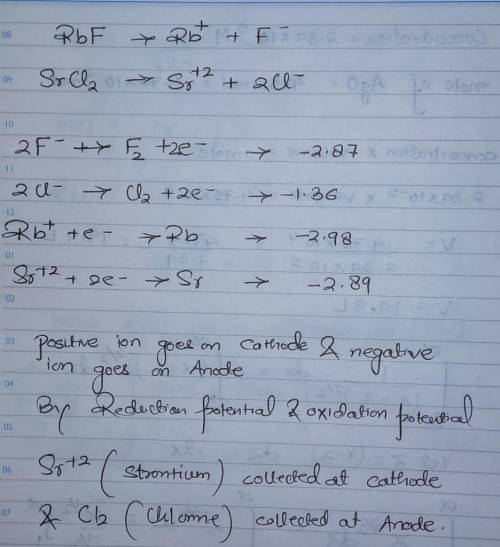Chemistry, 06.05.2020 00:22, osmanysalvador9
In the electrolysis of a molten mixture of RbF and SrCl2, identify the product that forms at the negative electrode and at the positive electrode. The cell temperature must be high enough to keep the salt mixture molten hence the metal appears as a liquid and the halogen as a gas. product at the negative electrode (cathode) product at the positive electrode (anode)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, ayoismeisjjjjuan
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1


Chemistry, 23.06.2019 01:30, Dmoney5104
The biomedical technique in which a part of the brain is destroyed with electric current is known as a. electroconvulsive therapy b. prefrontal lobotomy c. bilateral cingulotomy d. tardive dyskinesia
Answers: 2

Chemistry, 23.06.2019 04:00, Tiredd7838
Which of these are physical changes in matter? check all that apply boiling water a pencil being sharpened exploding dynamite freezing water rotting cheese
Answers: 1
Do you know the correct answer?
In the electrolysis of a molten mixture of RbF and SrCl2, identify the product that forms at the neg...
Questions in other subjects:



Mathematics, 26.06.2020 23:01




Mathematics, 26.06.2020 23:01

Mathematics, 26.06.2020 23:01

Mathematics, 26.06.2020 23:01