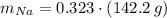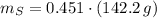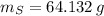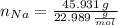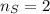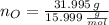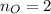Chemistry, 06.05.2020 02:23, BrodsterBj
Calcula la fórmula molecular de un compuesto formado por 32.3% de sodio, 45.1% de azufre y 22.5% de oxígeno, el peso molecular del compuesto es de 142.2 gr

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:50, giiffnlojd
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3


Chemistry, 22.06.2019 18:10, NEONREDBLADE
Areader can tell that the meaning of “obnoxious” will include “having the quality of something” because of the .a) prefix b)pronunciation c)suffix d) word root
Answers: 3

Do you know the correct answer?
Calcula la fórmula molecular de un compuesto formado por 32.3% de sodio, 45.1% de azufre y 22.5% de...
Questions in other subjects:











 .
.