
A chemist designs a galvanic cell that uses these two half-reactions:
O2 (g) + 4H+(aq) + 4e− → 2H2O (l) Eo =+1.23V
Zn+2 (aq) + 2e− → Zn(s) Eo=−0.763V
Answer the following questions about this cell.
Write a balanced equation for the half-reaction that happens at the cathode.
Write a balanced equation for the half-reaction that happens at the anode.
Write a balanced equation for the overall reaction that powers the cell. Be sure the reaction is spontaneous as written. Do you have enough information to calculate the cell voltage under standard conditions

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, anthony4034
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1


Chemistry, 22.06.2019 20:00, AaronEarlMerringer
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1

Chemistry, 22.06.2019 22:30, robertss403
How many moles of kci are produced from 2.50 moles k
Answers: 1
Do you know the correct answer?
A chemist designs a galvanic cell that uses these two half-reactions:
O2 (g) + 4H+(aq) +...
O2 (g) + 4H+(aq) +...
Questions in other subjects:


Mathematics, 07.09.2021 07:50

Mathematics, 07.09.2021 07:50

Engineering, 07.09.2021 07:50

Mathematics, 07.09.2021 07:50




Mathematics, 07.09.2021 07:50

Mathematics, 07.09.2021 07:50


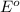 reduction potential will always get reduced and will undergo reduction reaction.
reduction potential will always get reduced and will undergo reduction reaction. 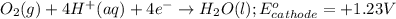
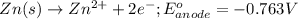 ( × 2)
( × 2)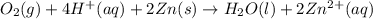
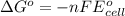
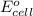 of the reaction, we use the equation:
of the reaction, we use the equation: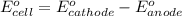
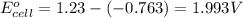
![E_{cell}=E^o_{cell}-\frac{0.059}{n}\log \frac{[Zn^{2+}]^2}{[H^{+}]^4\times p_{O_2}}](/tpl/images/0645/2279/2e91a.png)




