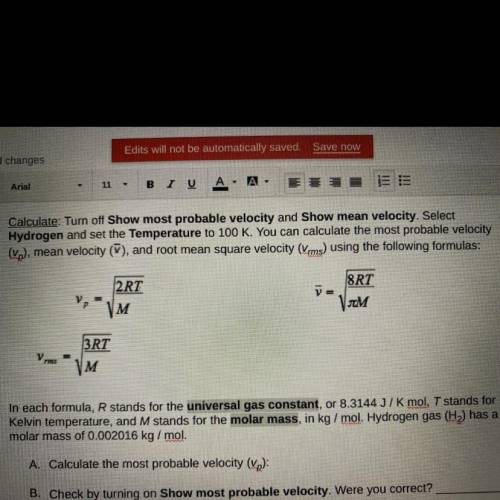
In each formula, R stands for the universal gas constant, or 8.3144 J/K mol, T stands for
Kelv...

Chemistry, 06.05.2020 02:14, vandarughb5653
In each formula, R stands for the universal gas constant, or 8.3144 J/K mol, T stands for
Kelvin temperature, and M stands for the molar mass, in kg/mol. Hydrogen gas (H) has a
molar mass of 0.002016 kg/mol.
A. Calculate the most probable velocity (vp):
B. Check by turning on Show most probable velocity. Were you correct?
C. Calculate the mean velocity (V):
D. Check by turning on Show mean velocity. Were you correct?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, toledanomariap43bxm
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3

Chemistry, 22.06.2019 19:50, mikaylaaaaa
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 23.06.2019 15:30, tymiahill7244
Iv the concentration of ionic substances is important for the heart to beat. your heart responds to electrical impulses that travel through heart cells that are made up mostly of water. which properties of ionic compounds are important to support this function? solubility in water conductivity crystalline melting point الم" الا به done رلرلرللللہ و و او 8
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Biology, 27.01.2020 21:31

Chemistry, 27.01.2020 21:31



Mathematics, 27.01.2020 21:31

English, 27.01.2020 21:31

History, 27.01.2020 21:31

English, 27.01.2020 21:31

History, 27.01.2020 21:31







