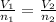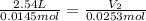Chemistry, 06.05.2020 02:01, leyslebravo2729
0.0145 moles of helium gas are introduced into a balloon so that the volume of the balloon is 2.54 liters. An additional amount of helium is added to the balloon so that the total amount of helium is 0.0253 moles. Calculate the final volume of the balloon.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:20, sindy35111
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l. s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3

Chemistry, 22.06.2019 13:30, kkingstone7062
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1

Chemistry, 22.06.2019 16:00, hjgjlgkjg
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1
Do you know the correct answer?
0.0145 moles of helium gas are introduced into a balloon so that the volume of the balloon is 2.54 l...
Questions in other subjects:



Social Studies, 05.05.2020 20:20

Mathematics, 05.05.2020 20:20

Chemistry, 05.05.2020 20:20

Chemistry, 05.05.2020 20:20


English, 05.05.2020 20:20

English, 05.05.2020 20:20

Chemistry, 05.05.2020 20:20