
Methanol, is made by using a catalyst to react carbon monoxide with hydrogen at high temperature and pressure. Assuming that 450.0 of CO and 825 of H2O are allowed to react, answer the following questions. (Hint: First write the balanced chemical equation for this reaction.)
Which reactant is in excess?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, ashlpiriz123
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 16:50, briansalazar17
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 21:00, alwaysneedhelp84
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1

Chemistry, 23.06.2019 00:00, bryn2433
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3
Do you know the correct answer?
Methanol, is made by using a catalyst to react carbon monoxide with hydrogen at high temperature and...
Questions in other subjects:

English, 26.11.2020 15:30


English, 26.11.2020 15:30


World Languages, 26.11.2020 15:30


English, 26.11.2020 15:40

Computers and Technology, 26.11.2020 15:40

Mathematics, 26.11.2020 15:40

Physics, 26.11.2020 15:40


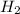 are allowed to react, answer the following questions. (Hint: First write the balanced chemical equation for this reaction.)
are allowed to react, answer the following questions. (Hint: First write the balanced chemical equation for this reaction.)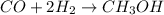
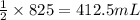 of carbon monoxide
of carbon monoxide



