
Chemistry, 06.05.2020 05:15, ayoismeisalex
A solid weak acid is weighed, dissolved in water and diluted to exactly 50.00 ml. 25.00 ml of the solution is taken out and is titrated to a neutral endpoint with 0.10 m NaOH. The titrated portion is then mixed with the remaining untitrated portion and the pH of the mixture is measured.
Mass of acid weighed out (grams) 0.755
Volume of NaOH required to reach endpoint: (ml) 18.8
pH of the mixture (half neutralized solution) 3.51
1. What is the pKa of the acid?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, andaws21
This large tectonic plate is bounded on three sides by whats know as the ring of fire. what is the name of this tectonic plate? a) pacific plate b) eurasian plate c) north american plate d) indo- australian plate plz it's science but there's no option for science so i picked chemistry
Answers: 2



Chemistry, 22.06.2019 14:30, emilymartinez75
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1
Do you know the correct answer?
A solid weak acid is weighed, dissolved in water and diluted to exactly 50.00 ml. 25.00 ml of the so...
Questions in other subjects:





Mathematics, 12.02.2021 22:20


Biology, 12.02.2021 22:20

Arts, 12.02.2021 22:20

Mathematics, 12.02.2021 22:20



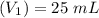
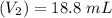
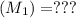
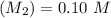
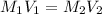
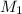 the subject of the formula; we have:
the subject of the formula; we have: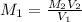
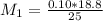
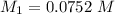
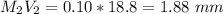
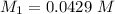
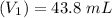
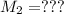
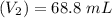
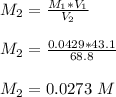
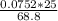
![pH = pKa + log \frac{[salt]}{[acid]}](/tpl/images/0646/6370/2dc0b.png)
![3.51= pKa + log \frac{[0.0273]}{[0.0273]} \\ \\ 3.51= pKa + log \ 1 \\ \\ 3.51= pKa + 0 \\ \\ pKa = 3.51](/tpl/images/0646/6370/56a03.png)




