

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, bionicboy03120440
What is the mass of sodium in 3 moles of sodium chloride
Answers: 1

Chemistry, 22.06.2019 20:40, ohgeezy
Select the correct value for the indicated bond angle in each of the compounds. o−o−oo−o−o angle of o3 90° 109.5° < 109.5° 120° < 120° 180° f−b−ff−b−f angle of bf3 180° < 109.5° < 120° 120° 109.5° 90° f−o−ff−o−f angle of of2 < 120° 120° 90° 109.5° 180° < 109.5° cl−be−clcl−be−cl angle of becl2 90° 109.5° 180° 120° < 109.5° < 120° f−p−ff−p−f angle of pf3 90° 109.5° < 109.5° 180° 120° < 120° h−c−hh−c−h angle of ch4 90° < 109.5° 180° 120° < 120° 109.5°
Answers: 1


Chemistry, 23.06.2019 01:00, bsheepicornozj0gc
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1
Do you know the correct answer?
Glycine has pKa values of 2.34 and 9.60. Therefore, glycine will exist predominately as a zwitterion...
Questions in other subjects:


Mathematics, 21.04.2020 03:09

Mathematics, 21.04.2020 03:09


Mathematics, 21.04.2020 03:09





Mathematics, 21.04.2020 03:09


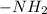 and -COOH should bear equal and opposite charges in it's zwitterionic structure.
and -COOH should bear equal and opposite charges in it's zwitterionic structure.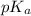 of -COOH (in glycine) = 2.34
of -COOH (in glycine) = 2.34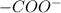 .
.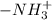 .
.



