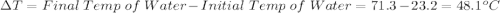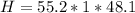Chemistry, 06.05.2020 06:05, fdougie111
You are running a calorimetry experiment where you are trying to determine the number of Calories (with a capital C!) in a peanut. You set up your aluminum can of water and take all your initial data, putting it in the table below. Then, you set your peanut ON FIRE You finish filling out your table once the peanut has gone out. How many Calories of heat did your peanut release? Round your answer to two digits after the decimal point.
Initial Mass of Peanut 3.11 grams
Final Mass of Peanut 0.52 grams
Mass of Water 55.2 grams
Initial Temp of Water 23.2 degrees C
Final Temp of Water 71.3 degrees C

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 11:30, claudr03
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3

Chemistry, 22.06.2019 18:10, sangamlama
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
Do you know the correct answer?
You are running a calorimetry experiment where you are trying to determine the number of Calories (w...
Questions in other subjects:


Mathematics, 28.01.2020 02:31





Geography, 28.01.2020 02:31



Health, 28.01.2020 02:31


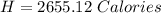
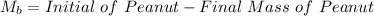
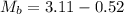
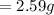
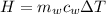
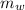 is the mass of water which is given as
is the mass of water which is given as 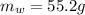
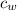 is the specific heat of water which has a constant value of
is the specific heat of water which has a constant value of 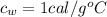
 is the change in temperature which can be evaluated as follows
is the change in temperature which can be evaluated as follows