
Standard solution of FeSCN2+FeSCN2+ is prepared by combining 9.09.0 mL of 0.200.20 M Fe(NO3)3Fe(NO3)3 with 1.01.0 mL of 0.00200.0020 M KSCN. KSCN. The equation for the reaction is as follows. Fe(NO3)3+KSCN↽−−⇀FeSCN2++KNO3+2NO−3 Fe(NO3)3+KSCN↽−−⇀FeSCN2++KNO3+2NO3− What allows us to assume that the reaction goes essentially to completion? The reaction quotient Q is greater than Kc. Kc. The concentration of Fe(NO3)3Fe(NO3)3 is much higher than the concentration of KSCN. KSCN. The excess Fe3+Fe3+ prevents the formation of the neutral Fe(SCN)3.Fe(SCN)3. The equlibrium reaction has a very high Kc. Kc. Under the conditions given, Le Châtelier's principle dictates that the reaction shifts to the left. Based on that assumption, what is the equilibrium concentration of FeSCN2+?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:30, annanikherrera
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 22.06.2019 14:00, Killion2022
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1
Do you know the correct answer?
Standard solution of FeSCN2+FeSCN2+ is prepared by combining 9.09.0 mL of 0.200.20 M Fe(NO3)3Fe(NO3)...
Questions in other subjects:










English, 04.05.2021 01:00


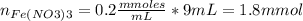
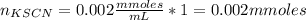
![[Fe(NO3)3]=\frac{1.8}{10} =0.18M](/tpl/images/0646/9896/2543f.png)
![[KSCN]=\frac{0.002}{10} =0.0002M](/tpl/images/0646/9896/5abd0.png)




