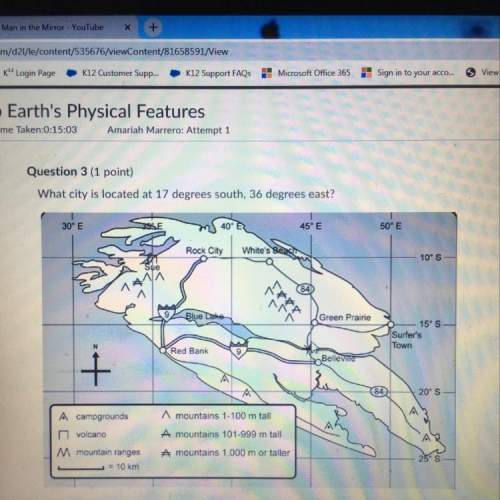
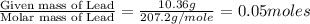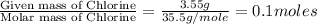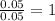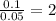Chemistry, 06.05.2020 07:29, jalexyinez
In an experiment similar to the zinc chloride experiment, a student placed a piece of lead in hydrochloric acid. Hydrogen gas was given off, and then the liquid was boiled off. The remaining solid, lead chloride was massed. Use the data below to determine the empirical formula of lead chloride.
mass of beaker 204.35 g
mass of lead and beaker before reaction 214.71 g
mass of lead chloride and beaker after reaction 218.26 g

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, rebeccacruzz2017
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 17:00, Estrella2209
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 23.06.2019 02:30, erikacastro259
what is your question? collegechemistry 5+3 pts in november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 3

Chemistry, 23.06.2019 05:40, Queenquestion9130
Why is any chemical reaction always balanced? give reasons and explain the easiest way to solve the balancing problems in chemical equations with stoichiometric coefficients upto 20 as hit and trial doesn't always work. give full reasoning
Answers: 1
Do you know the correct answer?
In an experiment similar to the zinc chloride experiment, a student placed a piece of lead in hydroc...
Questions in other subjects:




Mathematics, 01.09.2020 14:01



Physics, 01.09.2020 14:01




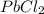
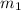 = 204.35 g
= 204.35 g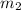 = 214.71 g
= 214.71 g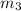 = 218.26 g
= 218.26 g = [214.71 - 204.35] = 10.36 g
= [214.71 - 204.35] = 10.36 g = [218.26 - 214.71] = 3.55 g
= [218.26 - 214.71] = 3.55 g