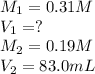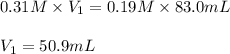Chemistry, 06.05.2020 07:23, MarMarMar07
A student needs to dilute a 0.31 M Pb(NO3)2 solution to make 83.0 mL of 0.19 M Pb(NO3)2 . Set up the calculation by placing the values with the correct units into the equation. Then, calculate the volume, in milliliters, of the 0.31 M Pb(NO3)2 solution that is needed.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:50, bridgetosanders
What are four significant sources of ghgs that come from wostem washington?
Answers: 2


Chemistry, 22.06.2019 19:30, simihehe
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 23.06.2019 05:40, shelbylynn1093
Why is it incorrect to balance a chemical equation by changing the subscripts? explain.
Answers: 2
Do you know the correct answer?
A student needs to dilute a 0.31 M Pb(NO3)2 solution to make 83.0 mL of 0.19 M Pb(NO3)2 . Set up the...
Questions in other subjects:

Mathematics, 23.04.2021 18:50

Mathematics, 23.04.2021 18:50

Mathematics, 23.04.2021 18:50


Biology, 23.04.2021 18:50

Mathematics, 23.04.2021 18:50

English, 23.04.2021 18:50




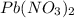 solution that needed is, 50.9 mL
solution that needed is, 50.9 mL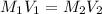
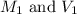 are the initial molarity and volume of dilute
are the initial molarity and volume of dilute 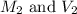 are the final molarity and volume of
are the final molarity and volume of