A chemist measures the energy change Delta H during the following
2Fe2O3(s) > FeO(s) + O2(g...

Chemistry, 06.05.2020 07:23, davelopez979
A chemist measures the energy change Delta H during the following
2Fe2O3(s) > FeO(s) + O2(g). ΔH = 560 kJ
1) This reactions is .
O Endothermic
O Exothermic
2) Suppose 94.2g of Fe2O3 react. will any heat be relased or absorbed?
O Yes, absorbed.
O Yes, released.
O No.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, natalie857123
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 22.06.2019 10:00, halohero7
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 15:00, MilanPatel
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 18:10, bri9263
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3
Do you know the correct answer?
Questions in other subjects:

Physics, 07.01.2020 09:31

Social Studies, 07.01.2020 09:31

History, 07.01.2020 09:31

Mathematics, 07.01.2020 09:31


Mathematics, 07.01.2020 09:31

Mathematics, 07.01.2020 09:31

Mathematics, 07.01.2020 09:31

Mathematics, 07.01.2020 09:31


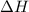 for the reaction comes out to be positive.
for the reaction comes out to be positive.




