Chemistry, 06.05.2020 07:22, babyduckies37
The contents of a rock have a 206Pb to 238U mass ratio of 0.135:1.00. Assuming that the rock did not contain any 206Pb at the time of its formation, determine the age of the rock. Uranium-238 decays to lead-206 with a half-life of 4.5 billion years. Express the time to two significant digits.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:30, bionicboy03120440
What is the mass of sodium in 3 moles of sodium chloride
Answers: 1


Chemistry, 23.06.2019 08:00, mshields1994
Amechanical wave that transports a lot of energy will have a
Answers: 2
Do you know the correct answer?
The contents of a rock have a 206Pb to 238U mass ratio of 0.135:1.00. Assuming that the rock did not...
Questions in other subjects:


SAT, 16.10.2021 18:10




Chemistry, 16.10.2021 18:10

Mathematics, 16.10.2021 18:10




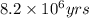
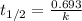
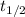 = half life of the reaction = 4.5 billion years =
= half life of the reaction = 4.5 billion years = 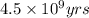
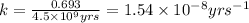
![k=\frac{2.303}{t}\log\frac{[A_o]}{[A]}](/tpl/images/0647/7147/f1041.png)
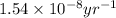
![[A_o]](/tpl/images/0647/7147/dc622.png) = initial amount of the sample = [1.00 + 0.135] = 1.135 g
= initial amount of the sample = [1.00 + 0.135] = 1.135 g