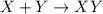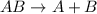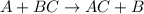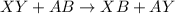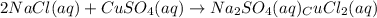Chemistry, 06.05.2020 07:18, s3r3naagarc1a
This equation represents a:
A. synthesis reaction.
B. decomposition reaction.
C. single replacement reaction.
D. double replacement reaction

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:20, tenleywood
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 16:10, 00015746
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
Do you know the correct answer?
This equation represents a:
A. synthesis reaction.
B. decomposition reaction.<...
A. synthesis reaction.
B. decomposition reaction.<...
Questions in other subjects:

Mathematics, 05.05.2020 14:34

History, 05.05.2020 14:34


Mathematics, 05.05.2020 14:34




History, 05.05.2020 14:34

Mathematics, 05.05.2020 14:34

Mathematics, 05.05.2020 14:34