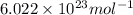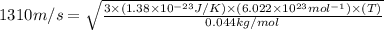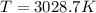Suppose that the root‑mean‑square velocity vrms of carbon dioxide molecules (molecular mass is equal to 44.0 g/mol ) in a flame is found to be 1310 m/s. What temperature T does this represent? The Boltzmann constant is k=1.38×10−23 J/K and Avogadro's number is A=6.022×1023 mol−1.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, KnMcdonaldk93906
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1


Chemistry, 22.06.2019 02:50, JuniperGalaxy
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 07:20, mathman783
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1
Do you know the correct answer?
Suppose that the root‑mean‑square velocity vrms of carbon dioxide molecules (molecular mass is equal...
Questions in other subjects:


Physics, 27.02.2021 06:40

Mathematics, 27.02.2021 06:40

Business, 27.02.2021 06:40

Biology, 27.02.2021 06:40

Health, 27.02.2021 06:40



Physics, 27.02.2021 06:40

English, 27.02.2021 06:40


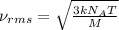
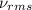 = root mean square speed = 1310 m/s
= root mean square speed = 1310 m/s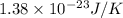
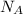 = Avogadro’s number =
= Avogadro’s number =