
Chemistry, 06.05.2020 07:13, maddy3lizabeth
A student makes a saturated sugar solution by dissolving sugar grains in warm water, stirring, and adding more sugar until it no
longer dissolves. The student then allows the solution to come to room temperature. The student pours equal volumes of the sugar
solution into five beakers. The student places a string into each of the beakers, with one end of the string hanging free outside of
the glassware. The student then places the beakers into five chambers of varying temperature (5, 10, 15, 20, and 25 degrees
Celsius). The student allows the beaker and string to sit for two weeks. At the end of the experiment, the student notices crystals
have formed in the string. The student masses the amount of crystals that have formed from the sugar solution on the string.
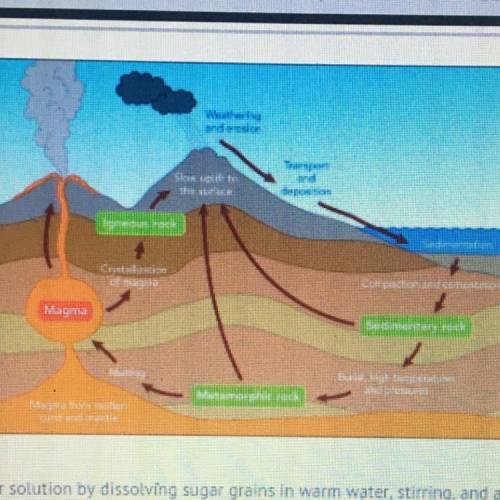
Select the dependent variable in this experiment and what process in the rock cycle is the student most likely modeling?
A-The dependent variable is the level of crystal formation and the student is
modeling the formation of igneous rock from magma.
B-The dependent variable is the level of crystal formation and the student is
modeling the melting of metamorphic rock to form magma.
C-The dependent variable is the temperature at which crystals form and the
student is modeling the formation of igneous rock from magma.
D-The dependent variable is the temperature at which crystals form and the
student is modeling the melting of metamorphic rock to form magma.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, jeffcarpenter
Write a net ionic equation to show that hydrofluoric acid, behaves as an acid in water.
Answers: 1

Chemistry, 22.06.2019 09:00, krystalhurst97
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 11:40, arlabbe0606
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3

Chemistry, 22.06.2019 15:30, dylannhandy
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2
Do you know the correct answer?
A student makes a saturated sugar solution by dissolving sugar grains in warm water, stirring, and a...
Questions in other subjects:





Mathematics, 21.12.2020 22:50

Chemistry, 21.12.2020 22:50


Arts, 21.12.2020 22:50

Mathematics, 21.12.2020 22:50

English, 21.12.2020 22:50






