
Chemistry, 17.10.2019 05:30, Blakemiller2020
All isotopes of a particular element have the same atomic number. how then do the isotopes of a particular element differ?
a. they have less or more protons.
b. they have less or more neutrons.
c. they have less or more electrons.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, asims13
Which element in the third period would you expect to have the larger atomic radius, sodium (na) or sulfur (s)? a. sodium, because it has a higher effective nuclear charge attracting electrons in fewer energy levels. b. sodium, because it has fewer protons attracting electrons in the same energy levels. c. sulfur, because it has more protons attracting electrons in more energy levels. d. sulfur, because it has a higher effective nuclear charge attracting electrons in the same energy levels.
Answers: 2


Chemistry, 22.06.2019 23:30, jade468
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1

Chemistry, 23.06.2019 00:00, tonimgreen17p6vqjq
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2
Do you know the correct answer?
All isotopes of a particular element have the same atomic number. how then do the isotopes of a part...
Questions in other subjects:



Mathematics, 27.10.2020 22:10



Mathematics, 27.10.2020 22:10

Mathematics, 27.10.2020 22:10

Mathematics, 27.10.2020 22:10

History, 27.10.2020 22:10

Health, 27.10.2020 22:10


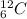 and
and 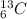 are isotopes. As both of them has 6 protons and there are 6 neutrons in
are isotopes. As both of them has 6 protons and there are 6 neutrons in 



