Chemistry, 26.04.2020 16:38, vicada2782
9. In the reaction of sodium hydroxide with chlorine gas, sodium chloride, sodium hypochlorite, and water a
reproduced. If 48.9g of chlorine gas is bubbled into a solution containing 54.2 g NaOH, how many grams
of NaClO can eventually be produced?
2 NaOH +Cl, = NaCl + NaClO + H, O

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:30, sanchez7489
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

Chemistry, 22.06.2019 18:30, kate3887
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1

Chemistry, 23.06.2019 03:00, Cheyenne7327
Analyze the reaction to determine whether the reaction is exothermic or endothermic. explain your reasoning.
Answers: 1
Do you know the correct answer?
9. In the reaction of sodium hydroxide with chlorine gas, sodium chloride, sodium hypochlorite, and...
Questions in other subjects:


History, 17.06.2020 23:57


Mathematics, 17.06.2020 23:57

History, 17.06.2020 23:57



Mathematics, 17.06.2020 23:57

Physics, 17.06.2020 23:57


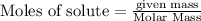
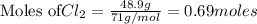
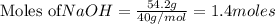
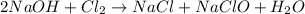
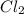 require = 2 moles of
require = 2 moles of 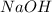
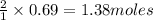 of
of 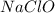
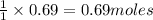 of
of