
Chemistry, 25.04.2020 23:51, gabrielolivas59
C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O (l)
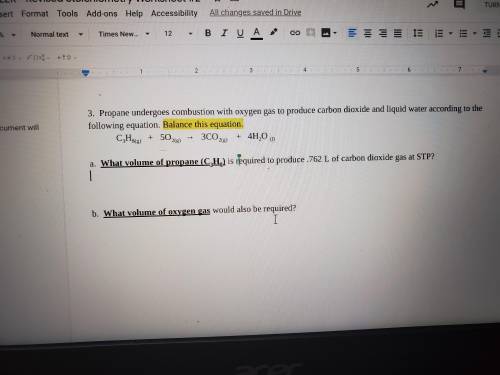
a. What volume of propane (C3H8) is required to produce .762 L of carbon dioxide gas at STP?
b. What volume of oxygen gas would also be required?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, annsmith66
What is the result of multiplying (2.5 × 1010) × (2.0 × 10-7)? a. 5.0 × 103 b. 5.0 × 10-3 c. 5.0 × 1017 d. 5.0 × 10-17
Answers: 1

Chemistry, 22.06.2019 05:30, alaynagrace1111
What is the mass of each element in a 324.8 sample of co2
Answers: 1


Chemistry, 22.06.2019 18:00, faithabossard
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3
Do you know the correct answer?
C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O (l)
a. What volume of propane (C3H8) is required to pr...
a. What volume of propane (C3H8) is required to pr...
Questions in other subjects:


Mathematics, 14.02.2021 03:10


Mathematics, 14.02.2021 03:10

Mathematics, 14.02.2021 03:10

English, 14.02.2021 03:10


Mathematics, 14.02.2021 03:10

Computers and Technology, 14.02.2021 03:10

Mathematics, 14.02.2021 03:10


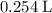 (at STP.)
(at STP.)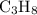 and
and 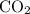 act like ideal gases.
act like ideal gases.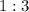 . As a result, for every
. As a result, for every 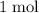 of
of 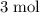 of
of 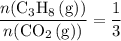 .
.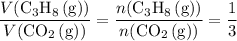 .
.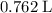 of
of 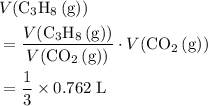 .
.



